Preimplantation Genetic Diagnosis (PGD) is a technique that enables the detection of genetic abnormalities in embryos before they are transferred to the uterus. It is a complementary technique to in vitro fertilisation (IVF), as it is through IVF that the embryos to be studied are obtained.
In 1990, the team led by Dr. Handyside published the first pregnancy achieved following PGD. Since then, the indications and diagnostic possibilities for PGD have expanded significantly.
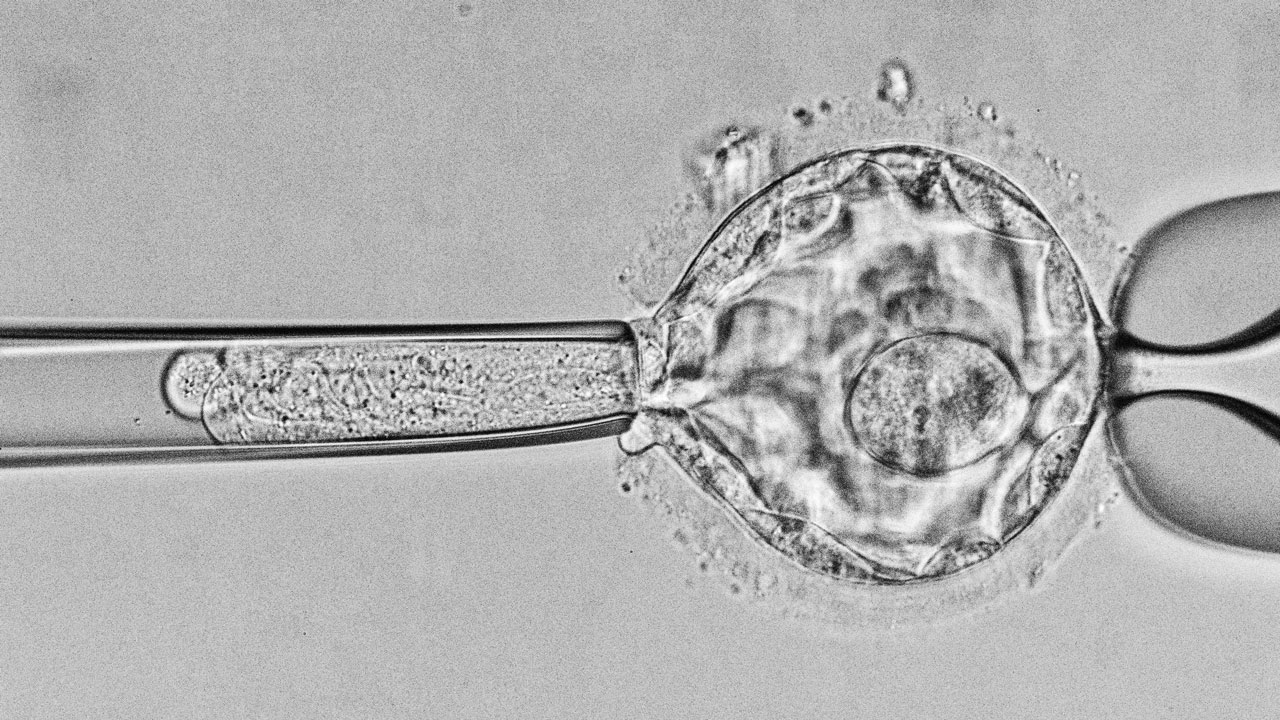
To carry out genetic analysis, a sample of the embryo must be obtained (embryo biopsy). This procedure can be performed at different stages of embryonic development: on Day 3 (when embryos typically have around 8 cells, one of which is extracted); or on Day 5 (when embryos have reached the blastocyst stage and a fragment of the trophectoderm is biopsied). The current trend is to perform the biopsy on Day 5, as the sample is more representative of the embryo as a whole. The extracted cell is analysed to detect certain genetic or chromosomal characteristics. While the study is in progress, if the biopsy was performed on Day 3, the embryos will be kept in culture in the laboratory. If the biopsy was performed on Day 5, the embryos will be frozen.
Embryos found to be genetically normal will be selected for transfer or freezing, while abnormal embryos will be discarded.
Thanks to PGD, the transmission of genetic and chromosomal diseases or abnormalities can be avoided, the risk of miscarriage is significantly reduced, and in some cases, the need for pregnancy termination is also avoided. Therefore, PGD increases the efficiency of IVF programmes.
In specific cases, before starting a PGD cycle, a consultation with a clinical geneticist is required. The geneticist will conduct an exhaustive study of the disease, define the procedures to be followed, and ensure accurate detection, as there are limitations to these techniques. It's also important to note that the success rate of treatment depends on each individual case.
The current legislation allows PGD in the following cases:
This technique can be useful in the following situations:
The biopsied cells are processed using a technique that amplifies the DNA to produce a sufficient amount for analysis. Two main PGD methods can be distinguished:
In some cases, an Informativity Study is required before starting the PGD cycle, in order to assess the diagnostic possibilities in each case.
PGD is highly reliable, but not 100 %. Therefore, once pregnancy is achieved, it is recommended to carry out a prenatal diagnostic test.
It is also important to consider how the patient responds to ovarian stimulation during the IVF cycle, which is necessary to obtain embryos for PGD. It is preferable to have several embryos available.
If the ovarian response is poor and few embryos are obtained, they may be frozen for use in future IVF cycles. Once a sufficient number of embryos are collected, they can be thawed and PGD can be performed on all of them.
In some cases, no normal embryos may be obtained.